For Carbon and Nitrogen Isotope Analysis with an Elemental Analyzer (e.g. biological tissues, plants)
1. Prepare the Sample Material for Isotopic Analysis
- Depending on the type of sample, a lipid extraction, demineralization, or acid fumigation step may be necessary. Remove water from your sample material using a drying oven or freeze dryer and then grind the sample material into a homogenous powder. Please e-mail the CSI if you are unsure about the proper way to prepare your sample material for isotope analysis.
2. Weigh/Seal Each Sample into a Tin Capsule

- To determine how much sample material to load into the tin capsule, you need to have a rough estimate of how much nitrogen and carbon is in your sample. For typical animal protein samples (~14 weight percent N and ~45 weight percent C), weights of 0.9-1.0mg are ideal, however, we routinely analyze protein samples on our Delta V mass spectrometer in the 0.5–0.6mg range. Samples with lower nitrogen concentrations, such as plants, will require larger amounts of sample, typically greater than 2 mg, but will be dependent on the specific type of sample. Once an appropriate amount has been determined, please keep the sample weights within a small range in order to minimize peak size variability. If you are unsure about the concentration of nitrogen or carbon in your samples, please e-mail the CSI for further instructions.
- Once the sample material has been loaded into the tin capsule, seal the capsule by crimping the open end and folding in all the edges of the capsule. Please make sure the sample material cannot leak out and that there are no thin edges of the capsule protruding. Thin, flat protrusions of capsule material can become stuck in the autosampler and cause sample loss. Forming the loaded capsule into a cube or sphere shape is recommended.
3. Prepare Samples for Shipment
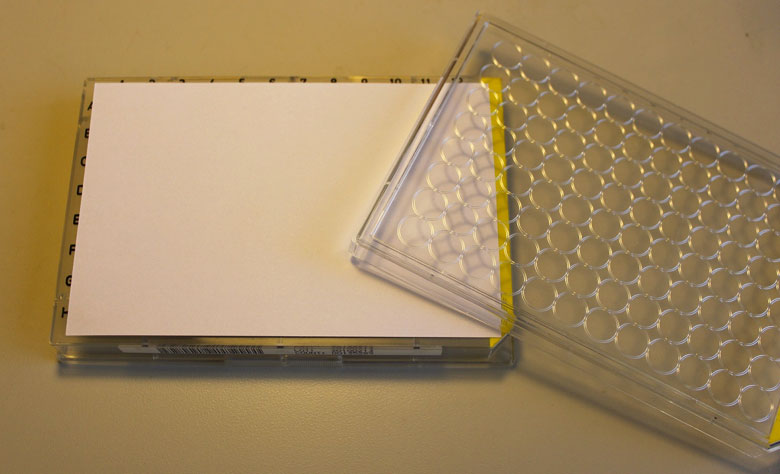
- The loaded capsules should be placed in a 96-well sample tray. If you are sending more than one type of sample material (e.g. bone collagen and muscle tissue), please organize your tray so that all samples of the same material are grouped together. If you have enriched your samples with an isotopic tracer, please order your samples from least enriched to most enriched (to the best of your ability), and be sure to indicate on the sample submission form that your samples are enriched.
- To ensure samples do not jump wells during shipment, place an index card on top of the open sample wells, under the lid. The card should cover all the open wells but should NOT be wedged tightly against the vertical edges of the lid, this could cause the index card to bow upward and allow the samples to jump wells.
- Secure the lid using tape and label each tray with your name, the type of samples, and a unique identifier (e.g. Tray 1, Tray 2).
For Hydrogen or Oxygen Isotope Analysis of Solid Materials with a High Temperature Conversion Elemental Analyzer (e.g. biological tissues)
- Follow steps 1-3 as for CN analysis above, except load samples into SILVER capsules. For hydrogen analyses of biological tissues with roughly 5-6% hydrogen, 0.2-0.3 mg sample weights are recommended. Please contact the CSI for recommened weights of all other materials.
For Carbon and Oxygen Isotope Analysis by Phosphoric Acid Digestion (e.g. carbonates, DIC)
- The amount of sample necessary depends on the type of material being analzyed. For d13C and d18O analysis of a PURE carbonate, 1 mg of material is sufficient. Samples with less than 100% carbonate content may require up to 20 mg of material for a good analysis. Solid samples should be sent to the CSI in well labeled, shatter-proof containers. For d13C of DIC in waters, please send at least 5 mL of each sample in a well labeled, well sealed container.
For Hydrogen and Oxygen Isotope Analysis of Waters
- Package each sample in a well labeled, well sealed container. Approximately 2 mL of sample are needed for analysis. If your samples have high concentrations of particulate matter, filtering may be necessary. For high salinity waters, please contact the CSI directly.
For All Other Isotope Analyses: Please contact the CSI directly
After All Samples Have Been Prepared: Fill out a Sample Submission Form and e-mail it to atudorei@unm.edu
- Fill out the Sample Submission Form spreadsheet, with sample ID’s and weights, must be submitted PRIOR to the arrival of your samples. Even if you send a paper copy of the form with your samples, an electronic copy is required. Do not hesitate to e-mail the CSI if you have any questions about the sample submission process.
MAIL To:
Dr. Viorel Atudorei
UNM Center for Stable Isotopes
221 Yale Blvd NE, Northrop Hall
MSC03-2040, 1 University of New Mexico
Albuquerque, NM, 87131 USA


